Large-sample hydrology is a critical field that addresses pressing global challenges, such as climate change, flood prediction, and water resource management. By leveraging vast datasets of hydrological and meteorological information across diverse regions, researchers develop models to predict water-related phenomena. This enables the creation of effective tools to mitigate risks and improve decision-making in real-world scenarios. These advancements are instrumental in safeguarding communities and ecosystems from water-related challenges.
A significant problem in hydrological research is the limited availability of datasets that support real-time forecasting and operational benchmarking. Traditional datasets like ERA5-Land, while comprehensive, are restricted to historical data, limiting their application in real-time forecasting. This restriction poses challenges for hydrological model development, as researchers cannot adequately test model performance under live conditions or evaluate how uncertainty in forecasts propagates through hydrological systems. These gaps hinder advancements in predictive accuracy and the reliability of water management systems.
Existing hydrological tools, such as CAMELS and ERA5-Land, provide valuable model development and evaluation insights. CAMELS datasets, which cover regions like the United States, Australia, and Europe, standardize data for various catchments and support regional hydrological studies. ERA5-Land, with its global coverage and high-quality surface variables, is widely used in hydrology. However, these datasets rely on historical observations and need more integration with real-time forecast data. This limitation prevents researchers from fully addressing the dynamic nature of water-related phenomena and responding effectively to real-time scenarios.
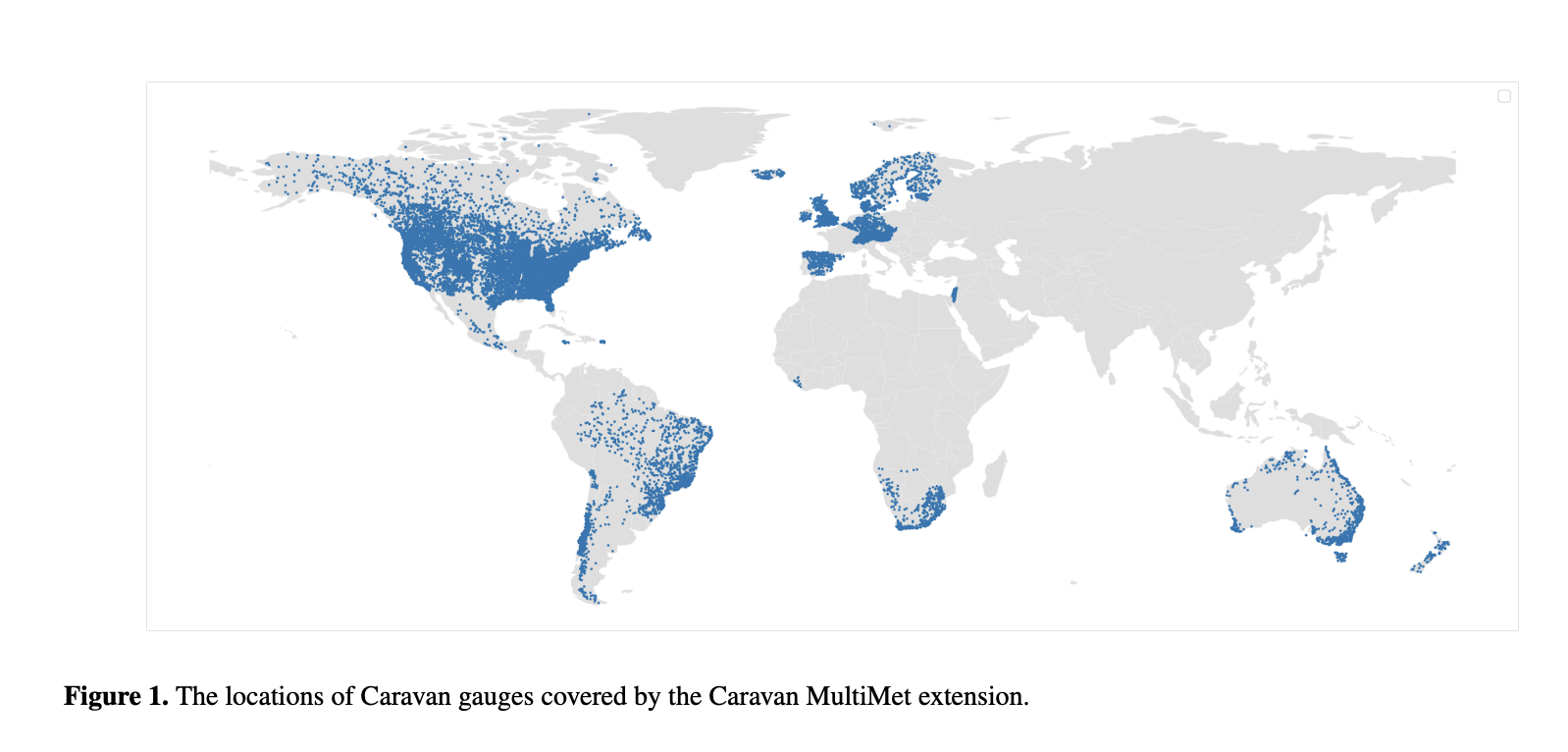
Researchers from Google Research introduced the Caravan MultiMet extension, significantly enhancing the existing Caravan dataset. This extension integrates six new meteorological products, including three nowcasts—CPC, IMERG v07 Early, and CHIRPS—and three weather forecasts—ECMWF IFS HRES, GraphCast, and CHIRPS-GEFS. These additions enable comprehensive analyses of hydrological models in real-time contexts. By incorporating weather forecast data, the extension bridges the divide between hindcasting and operational forecasting, establishing Caravan as the first large-sample hydrology dataset to include such diverse forecast data.
The Caravan MultiMet extension includes meteorological data aggregated at daily resolutions for over 22,000 gauges across 48 countries. The integration of both nowcast and forecast products ensures compatibility across datasets. For example, ERA5-Land data in the extension was recalculated in UTC zones to align with other products, simplifying comparisons. Forecast data, such as CHIRPS-GEFS, offers daily lead times ranging from one to 16 days, while GraphCast, developed by DeepMind, employs graph neural networks to produce global weather forecasts with a 10-day lead time. The extension’s zarr file format enhances usability, allowing researchers to efficiently query specific variables, basins, and periods without processing the entire dataset. Furthermore, including diverse spatial resolutions, such as CHIRPS’s high resolution of 0.05°, further enhances the dataset’s robustness for localized studies.
Including forecast data in Caravan has significantly improved model performance and evaluation capabilities. Tests revealed that variables such as temperature, precipitation, and wind components strongly agreed with ERA5-Land data, achieving R² scores as high as 0.99 in certain cases. For example, total precipitation data from GraphCast demonstrated an R² of 0.87 when compared to ERA5-Land, highlighting its reliability for hydrological applications. Similarly, ECMWF IFS HRES data showed compatibility with ERA5-Land variables, making it a valuable addition to the dataset. These results underscore the MultiMet extension’s effectiveness in enhancing hydrological models’ accuracy and applicability.
By introducing the Caravan MultiMet extension, researchers from Google Research addressed critical limitations in hydrological datasets. Integrating diverse meteorological products facilitates real-time forecasting, robust model benchmarking, and improved prediction accuracy. This advancement represents a significant step forward in hydrological research, enabling better water resource management and hazard mitigation decision-making. The availability of this dataset under open licenses further ensures its accessibility and impact on the global research community.
Check out the Paper and GitHub Page. All credit for this research goes to the researchers of this project. Also, don’t forget to follow us on Twitter and join our Telegram Channel and LinkedIn Group. If you like our work, you will love our newsletter.. Don’t Forget to join our 55k+ ML SubReddit.
[FREE AI VIRTUAL CONFERENCE] SmallCon: Free Virtual GenAI Conference ft. Meta, Mistral, Salesforce, Harvey AI & more. Join us on Dec 11th for this free virtual event to learn what it takes to build big with small models from AI trailblazers like Meta, Mistral AI, Salesforce, Harvey AI, Upstage, Nubank, Nvidia, Hugging Face, and more.
Nikhil is an intern consultant at Marktechpost. He is pursuing an integrated dual degree in Materials at the Indian Institute of Technology, Kharagpur. Nikhil is an AI/ML enthusiast who is always researching applications in fields like biomaterials and biomedical science. With a strong background in Material Science, he is exploring new advancements and creating opportunities to contribute.